Intelligent Oxygen to Optimize You on a Cellular Level
Breath by Breath
Making Oxygen simple and personalized
Physical Therapy
Elder Care
Home Health
Remote Field Operations
Product Not for Sale

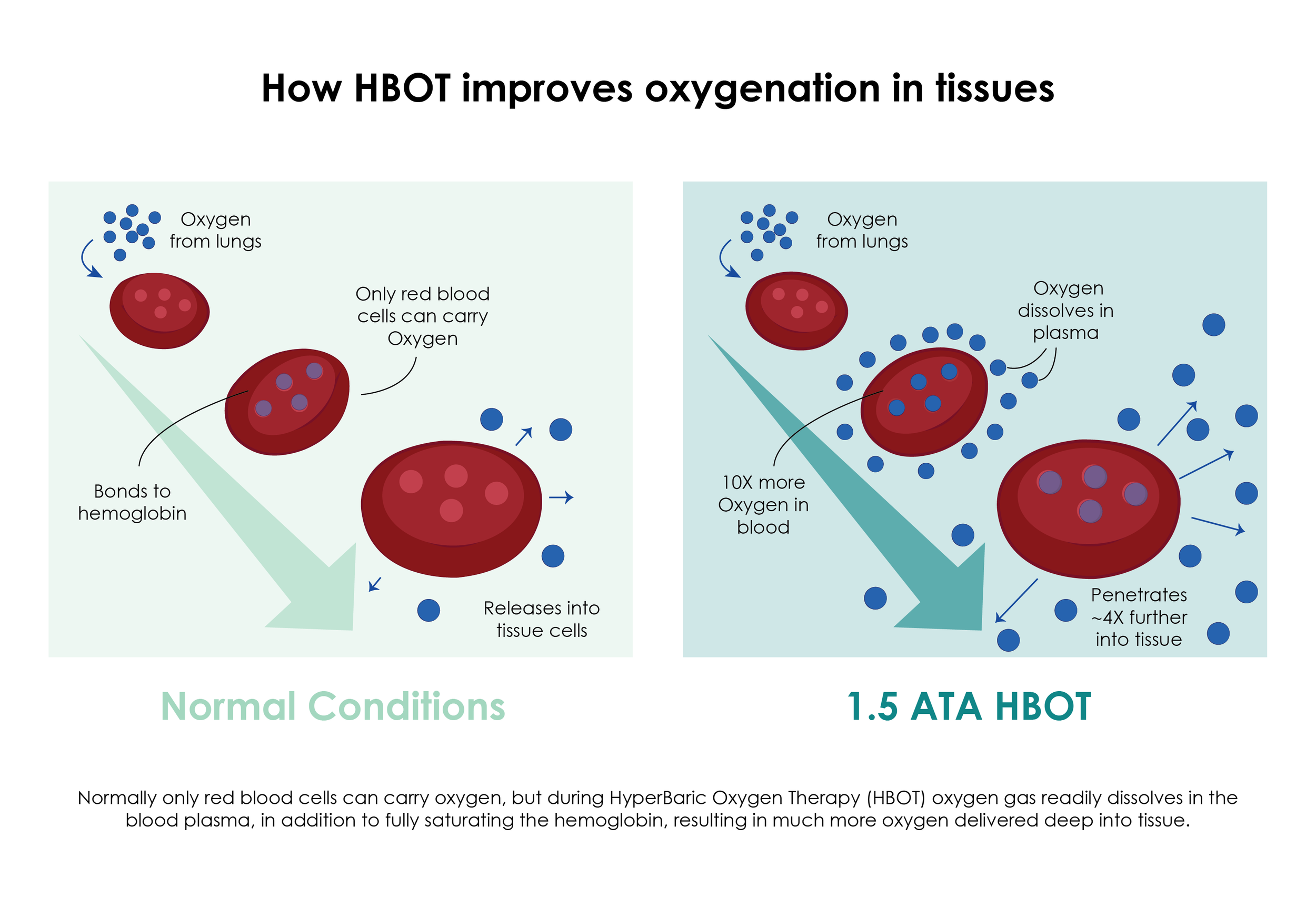
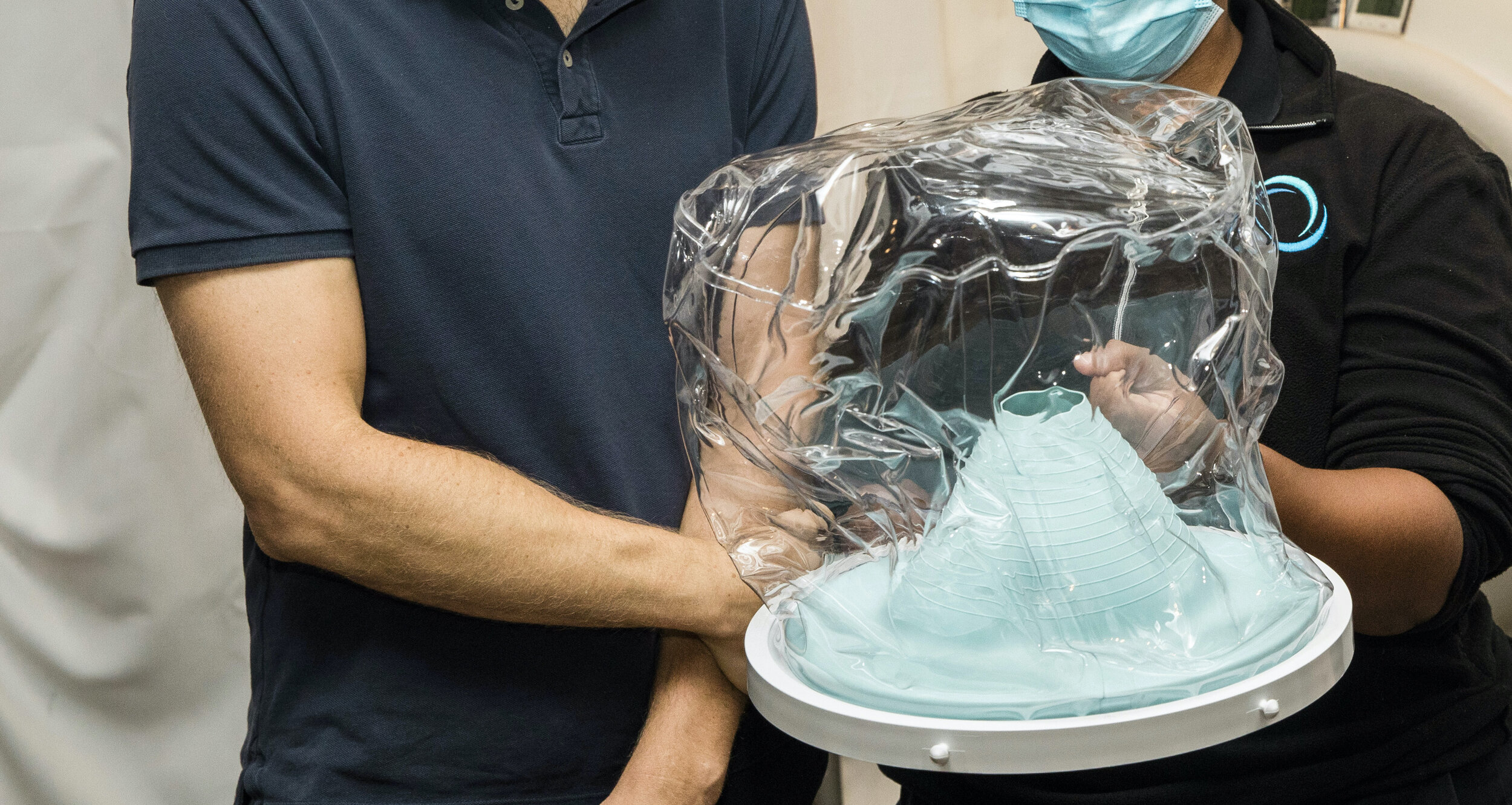
The AtmosThera soft hood system delivers oxygen therapy in a compact, noninvasive mobile form factor that can be delivered automatically at point of care, or in non-clinical settings such as home application. May be used together with other biometric devices to monitor, modify or guide therapy. Expected to prove useful in cases of Acute Respiratory Distress (ARDs), seen in many COVID-19 patients. The automated treatment process and integrated software system give it safe, reliable and simple operation even for home use. Patient outcomes will be correlated with precisely known treatment sessions.
THE TEAM
Alex Williams, Founder, COO
Visionary who founded Holistic Hyperbarics in 2017 where she remains CEO. (full bio)
Michelle Svatos, PhD, Founder, CEO
Serial medical device CEO, innovator & productizer, Adjunct Professor of Medical Physics at UW Madison & Dalhousie University (full bio)
Scott Sherr, MD, Founder, Medical Director
Worldwide Hyperbaric Medicine Expert & founder of Integrative Hyperbaric Medicine and Health Optimization. (full bio)
Adm Golub, Founder, Product Head
Photojournalist, Former Forest Service Hotshot Crew Sawyer and Wildland Firefighter, Builder of Things (full bio)
Tom Fox, MS, Founder, Technical Director
Research Physiologist & worldwide Hyperbarics technology expert (full bio)
Videos
Alex Williams talks about founding AtmosThera and managing an HBOT facility
AtmosThera’s Adm Golub interviews Dr. Scott Sherr, Medical Director, on uses of Hyperbaric Medicine and oxygen therapy

3 MAIN MARKET OPPORTUNITIES
$2B Proven respiratory ventilator market in 2019 Pre-COVID expected CAGR 4.7% thru 2022; adjusted to 7.9% in April 2020 Driver: non-invasive solutions for ARDs
$2B Hyperbaric O2 devices market $2B Key opportunities in brain: stroke and traumatic brain injury (TBI) Driver: Growing medical acceptance, cc indications·
$10B+ Home health, anti-aging, personal performance optimization market. Driver: usability, proven effectiveness, fit & finish
USE OF FUNDS
Fabricate, test, & benchmark minimum viable hardware
Web portal and phone app prototype for software
Build IP pipeline – submit further patents, sub-license in other fields
Map regulatory pathway with FDA consultants for initial offering
Map key clinical study to demonstrate effectiveness
3+ Key hires in Engineering and Program Management
IP US Patent Pending
FINANCIAL SNAPSHOT New Startup on ground floor – pre-funding and revenue
TECHNOLOGY STATUS Some component prototypes of core technology built, testing in progress. First product use cases, specifications and design well in progress.
CURRENT FUNDING OPPORTUNITY
$1.0 M seed round via Y-combinator SAFE notes (Simple Agreement for Future Equity), the standard Silicon Valley vehicle for early start-ups. Qualified Investors only.